Alphabet der Heimatkunde

Brass
Needles
Zinc
The museum of industrial-, economical- and social history "Zinkhütter Hof" in Stolberg is housed in a former glass factory which was erected in the 1830s. The authenticity and completeness of the well restored ensemble make the Zinkhütter Hof a very rare example of an architecture, commonly and typically used in the early phase of industrialization.
The buildings are grouped around an inner court yard and consist of a former glass factory an administration building and an additional building for the production of crucibles (melting pots), which were filled with sand, finely ground lime stone and a fluxing agent (for making the molten glass more fluid).
In the second half of the 19th century, production was closed down and shifted to a bigger, more modern plant built in the immediate neighbourhood. In 1904, the Zinkhütter Hof came into the possession of the later "Stolberger Zink", a company involved in mining as well as in zinc and lead production.
One part of the former factory building was rebuilt as an
impressive music hall. Later, the utilization changed again and
garages, a maintenance floor and laboratories were installed. No longer
bearing any resemblance to its original purpose, the factory building
became a mere wreck, a "Sleeping Beauty" for several decades.
In 1991, the entire site was bought by the town of Stolberg. With the
help of the federal state of North-Rhine-Westphalia and after extensive
restoration work, a new museum was established in the old plant. On
September 20th, 1996 the former glass factory was opened to the public
as Museum of Industrial, Economical and Social History in the Region of
Aachen.
The Zinkhütter Hof with its impressive set of historic buildings is not only an outstanding example of very early industrial architecture, but it is also located in a region which may well be considered as the origin of industrial development in Germany. Within a range of a few hundred yards, a whole chain of industrial plants was established during the first half of the 19th century:
Thus, in Stolberg the industrialization began at a very early time and a wide variety of natural resources favoured the development of the first industrial region in Germany, to which the rich deposits of local ore (zinc, lead, iron) as well as coal primarily contributed a great deal.
Roasting of sulphuric ores yielded sulphuric acid, which, together with locally exploited lime stone, served as basis for the production of soda. At those times, soda was a key product needed in large quantities in the process of making glass, for the production of detergents and in the textile industry.Thus, in Stolberg the industrialization began at a very early time and a wide variety of natural resources favoured the emergence of the very first industrial region in Germany. Especially the rich deposits of ore (zinc, lead, iron) as well as coal primarily contributed to this development. In other words, the Museum is located at a perfectly adequate location, namely at the very origin of industrial development in Germany.
Brass is an alloy (mixture) of the two metals copper and zinc,
consisting of approximately 70% copper and around 30% zinc. With
respect to colour, brightness and lustre, brass (when freshly polished)
bears a strong resemblance to gold. Not only for this reason our brass
exhibition was given the subtitle "The Gold of Stolberg". From the late
16th. until the end of the 18th. century, brass making was a "Golden
Trade" which brought the manufacturers in the valley of Stolberg
prosperity and wealth.
Nowadays, brass is made by melting together the two pure metals copper
and zinc. Until the late 18th.century, however, pure metallic zinc was
neither available nor even known. The alloy had thus to be made by
utilizing calamine, a zinc bearing ore, mostly consisting of
zinccarbonate or smithsonite.

Calamine, collection and
photo: F. Holtz, H. Wotruba
The geographic location of and the geologic situation in the region of Stolberg allowed brass production at a very early time. Whereas the local ores were already exploited and utilized for lead and iron smelting in celtic times, brass making was obviously started by the Romans in the first century.
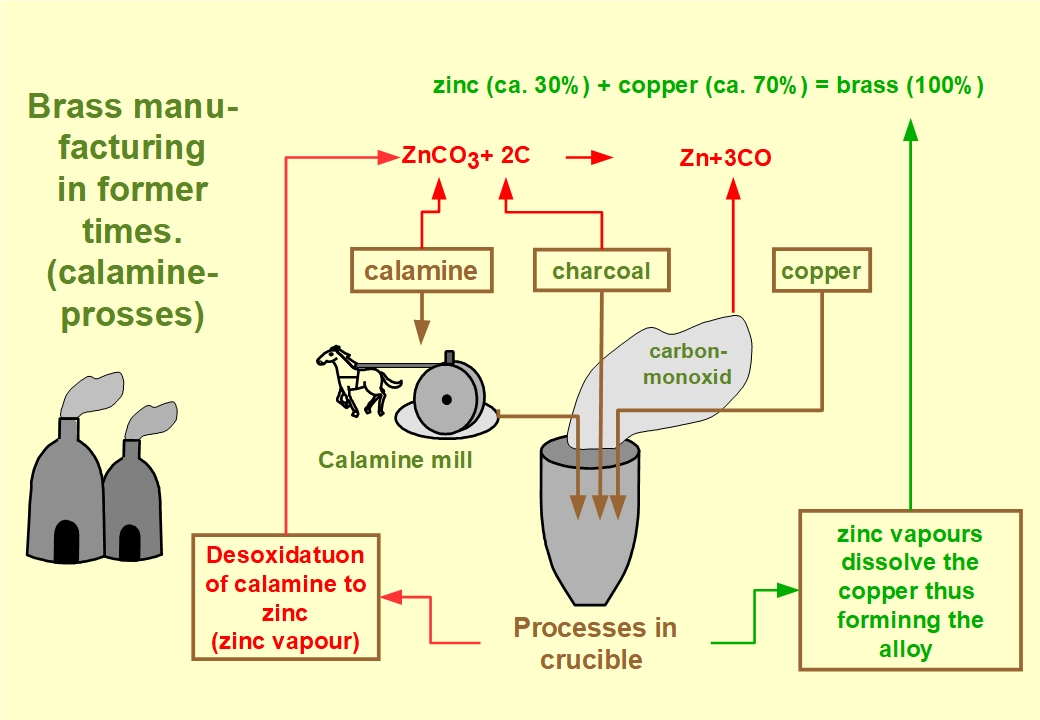
In the archaic process of brass making, crushed and pulverized calamine as well as pulverized charcoal together with pieces of copper were given into crucibles. At temperatures between 900 and nearly 1000 Degrees Centigrade the carbonate of the charcoal reacted with the oxygen of the ore, since air was not available in the crucible. The blaze in the furnace prevented the oxygen content of air, which is necessary for burning, to reach the charcoal. This process is commonly termed reduction (deoxydation), meaning that the calamine ore is reduced to zinc. Or more generally: The metallic zinc is separated from the ore.
Since temperatures between 900 and nearly 1000 Degrees Centrigrad required for this process, were well above the evaporation point of the zinc this metall was set free as zinc vapours. These zinc vapours dissolved the still solid copper, thus forming the alloy.
In this process, the required quantity of zinc ore was twice as high as the required copper weight. Consequently, brass making was almost exclusively restricted to areas with calamine deposits, as we had in Stolberg.
Sizze F. Holtz
The region of Aachen is considered as one of the most important centrers of needle production in Germany and one of the last still existing. The needles manufactured in Aachen were held in high esteem not only in Germany but also worldwide. In our museum we show machines and tools documenting the different procedures necessary to produce a needle, starting with a piece of wire and ending with the final check of the finished product.
Due to many different requirements of a highly specialized textile industry, a wide variety of complex needle designs had to be developed to meet the particular demands of industry. The recorded history of needle making in Aachen reaches back to the 16th century. Whereas a listing of guild members dates back to 1584, a first set of guild rules was drafted in 1615. Among others, these rules specified that only pure and best quality steel wires had to be used for needle making.
In 1798, Napoleon abolished the guilds and the opening of the
French
market resulted in a temporary bloom of needle manufacturing. During
the 19th century, numerous technological improvements were developed,
such as mechanical presses for shaping the eye-part of the needle and
punching the eye.
Around 1900, a total of 29 needle factories existed in Aachen employing
4022 workers.
In 1845, the American Elias Howe invented the first sewing machine and Isaak Merrit Singer (also an American) had another type of sewing machine patented in 1851. The method of using standardized and fully exchangeable parts which had been developed in the armour industry was successfully applied by Singer for mass-production of sewing machines. Customers were given the opportunity to pay by instalments. Thus, sewing machines were soon in common use, even in private households, and the needle manufacturers in Aachen started with the production of needles for sewing machines.
These needles for sewing machines were also made of a steel wire, having the same diameter as the base of the needle type to be produced. The diameter of the steel wire was partially reduced to form the slender needle shaft. A pair of hammering, rotating rams hit the wire along its circumference until the desired shaft diameter was reached (cold swagging). The machines had a capacity of 7 to 10 needle blanks per minute.
Subsequently the location, where the small hole for the sewing thread was to be punched, had to be flattened out as a precondition for punching, The second working step (punching) was made at a different station of the same machine.
After having punched the eye, another machine was used to cut
the
thread groove into the upper part of the shaft. During the processes of
pressing the eye part, punching the eye and cutting the thread groove,
sharp protruding edges were left, which had to be removed.
In order to do this, several needles were clamped into a pair of
pliers, the jaws of which could be moved sidewise and in opposite
direction to each other. In consequence, the clamped needles started to
rotate and could be ground on a grinding stone around their total
circumference.
Another machine was used to grind the needle points. A transport screw moved the needle ends over a grinding stone under the desired angle.
Between the different processes, the needles had to be washed
and dried in wooden, rotating barrels filled with saw dust.
For final inspection, the needles were adjacently placed in a row in
order to detect differences in length, surface properties and
appearance of the needle points. By applying very slight pressure a
finger was carefully moved over this row, thus causing all the
individual needles to rotate. In this way imperfect specimens could
easily be sorted out.
Until the end of the 18th century, zinc could not be made in its pure, metallic form and was not even known. As late as in the 1830s the metallurgists succeeded in producing zinc by using calamine, a special zinc ore which had previously been used in the brass making process for centuries.
At the beginning of the 19th century it was possible to smelt pure, metallic zinc on a large, industrial scale. In the meantime however, the ore deposits in the upper part of the local rock formations had mostly been exploited for brass making. Consequently, the miners had to go deeper to find sufficient quantities of ore. Upon reaching a certain depth, however, the characteristic of the ore changed from the calamine type to what we call “blende”, a sulphurous ore type mainly consisting of sphalerite.
Before processing this type of ore, it had to be roasted. This was necessary to set free the sulphur content in form of sulphur dioxide, which initially just escaped into the atmosphere. Together with the humidity of the air, this sulphur dioxide was transformed into sulphuric acid. At those times, even small patches of grass – let alone bushes or trees – were an absolute rarity in the neighbourhood of the smelteries.
Fortunately, some 15 years later special furnaces had been developed, which prevented the sulphur dioxide from escaping into the atmosphere. The sulphur dioxide was then utilized to produce soda (sodium carbonate Na2CO3). At that time, soda was a key product used for soap making and for manufacturing glass.
| Sketch: F. Holtz |
Prinziple of Zinc Distillation |
Coming back to the local ores, another particularity has to be mentioned: Where the local ore deposits had formerly been mined, the soil is highly contaminated with toxid lead-, zinc. and cadmium-minerals. In these locations an extremely specialized group of herbs has managed to adapt and to survive in this environment (Evolution). The most famous species of the aforementioned population of plants is a small, yellow blossomed violet, called viola lutea calamaria (Galmeinveilchen). It is a worldwide unique botanical speciality, which indeed can only be found in the neighbourhood of Stolberg (endemic).
The method of zinc smelting
Quite similar to brass making, the general problem in producing zinc
resulted from the fact, that the zinc content of the ore was not set
free as a liquid but as zinc vapour. In addition, the zinc vapour
instantly reacted with the oxygen of the air, thus forming zinc-oxide
powder. The problem was solved by condensing the zinc vapour under
complete exclusion of air.
The retorts (also called muffles) were placed within a furnace, where they could be heated to more than 1000 Degrees C. The muffles were filled with a mixture of pulverized zinc ore and pulverized coke. A condenser was firmly placed against the open end of the muffle and then the junction was covered with clay to avoid any leakages. The condenser thus protruded out of the hot furnace.
When the content of the muffle gradually heated up, zinc vapour together with carbon monoxide was set free, as was the case in brass making. When reaching the condenser, the gases cooled down and the zinc vapour condensed to liquid zinc, whereas the carbon monoxide streamed out of the other and open end of the condenser, thus keeping away the oxygen of the air. The liquid zinc collected in the condenser and could then be taken out by a special spoon and cast bars for rolling coils subsequently.
The carbon monoxide leaving the condenser was immediately ignited to form less dangerous carbon dioxide.
Normally the outlet of the condenser was elongated by a so called allonge. Small amounts of zinc vapour or microscopic droplets of liquid zinc which escaped from the condenser, solidified within the allonge. The resulting dust (either zinc or zinc oxide) collected at the inner walls of the allonge and could be utilized to enrich the next charge of ore.
Discharging and refilling of the muffles (called maneuver) was very, very hard work indeed. It had to be done under working conditions, we could not even imagine today. The replacement of a muffle for instance had to be done by hand; no lifters or auxiliary tools were available. And a muffle was not only heavy, it was also preheated to above 800oC.
Typically, the so called maneuver was started at 4 or 6 o’clock in the morning, depending on the season of the year. In other words, the extremely hard work was done during the cooler hours of the day. Even though the maneuver took 4 to 6 hours, the workers were paid for a complete 12 hours shift.